Demonstrations Page 1 - Assorted
Scroll down to view photographs and short descriptions of
some of the demonstrations in the list below.
WARNING – Use at your own risk! We cannot guarantee the accuracy or the safety of these activities. Some of these activities are far more dangerous than others. The contributors and Bradley University do not assume any responsibility for these activities or their results. If you have questions, corrections, or comments please do not hesitate to contact Dean Campbell (campbell@bumail.bradley.edu) at Bradley University.
- Ferrofluid
Demonstrations
- Refrigerator
Magnet Demonstrations
- Polydimethysiloxane
Demonstrations
- LEGO® Brick Chemistry and Nanotechnology Demonstrations
- No Suds! (water hardness)
- This is an easy demonstration if you live in a place like
the midwest where hard water is readily available. Acquire some
empty 20 oz. soda bottles and fill them halfway: one with hard
water, one with deionized water. Add the same amount of soap
to both bottle (several drops of inexpensive pine cleaner has
worked for me, Ivory soap shavings also work well) and close
the lids tightly on the bottles. Shake both bottles about 50
times. Immediately after shaking, the hard water will have more
foam on its surface, but this foam quickly breaks, leaving only
foam on the deionized (DI) water. The lack of foam on the hard
water is due the calcium and/or magnesium ions in the water.
These ions bind to the soap molecules in the water, rendering
them useless. One result of this is that little or no foam forms
on the surface of the water.

- References:
- Soriano, David S.; Draeger, Jon A. A water treatment experiment
(chemical hardness) for nonscience majors. J. Chem Educ.
1993 70 414.
- Birch, E. John H. Hardness in water- a demonstration. J.
Chem Educ. 1949 26 196.
-
- Demonstrating Poisson's Ratios with
Chicken Wire
- The lateral expansion or contraction of a material as it
is stretched can be bescribed by a mathematical relation called
the Poisson's ratio. Most materials tend to contract laterally
as they are stretched (and have a positive Poisson's ratio),
but some materials expand laterally as they are stretched (and
have a negative Poisson's ratio). Often a material that has a
positive Poisson's ratio can be modified to form a structure
that has a negative Poisson's ratio. Chicken wire can be used
to demostrate these ratios. Caution: Cut chicken wire can
be sharp!
- References:
- Campbell, D. J.;Querns, M. K. "Using Paper Cutouts to
Illustrate Poisson's Ratio." J. Chem. Educ., 2002,
79, 76.
- For instructions on how to make fullerene models out of chicken
wire, see Murphy, C. J.; Campbell, D. J. "A Chicken Wire
Buckyball." Chem. Educator 2000, 5,
1.
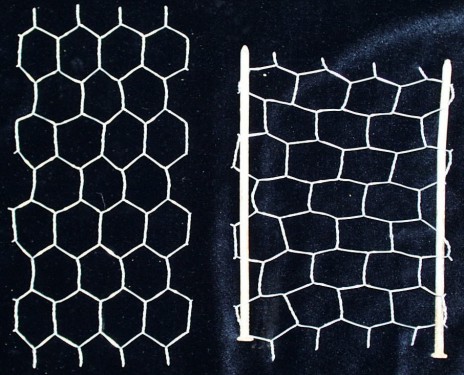
- ABOVE: Positive Poisson's ratio structures (LEFT) unstreched
and (RIGHT) stretched.
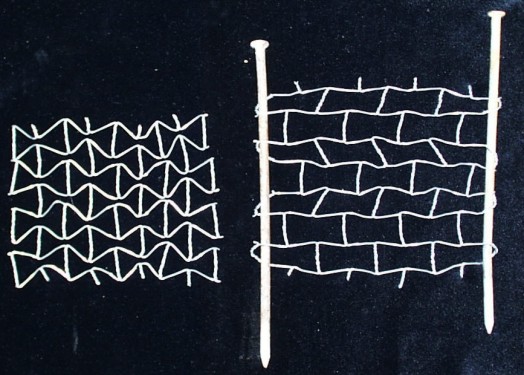
- ABOVE: Negative Poisson's ratio structures (LEFT) unstreched
and (RIGHT) stretched.
-
- Balloon in an Airplane
(air pressure)
- Most everyone who has traveled on an airplane has witnessed
some effects of decreased air pressure in the cabin of the plane:
ears "pop", poured soda fizzes a little more, and sealed
bags swell up. This last observed phenomenon was the basis of
a simple experiment run on a long flight from Chicago to Honolulu
to estimate the amount of air pressure change. The equipment
required is very simple: a typical rubber balloon, a flexible
measuring tape (such as one used for clothing measurements),
and a permanent felt-tip marker. All of this should present no
risk to airline security. Before the plane leaves the ground,
inflate the balloon about halfway. DO NOT fully inflate the balloon.
If it expands too much when the plane is in the air, the ballon
may pop. This not only ruins the experiment, but it may also
disturb fellow travel-weary passengers. The flexible measuring
tape is used to measure the circumference of the balloon. Since
most balloons are not perfectly spherical it is advisable to
use the marker to indicate the path of the measuring tape. That
way later measurements can be made across the same path. Once
set up, the balloon circumference can be measured at any time.
On the Chicago to Honolulu trip, a ground circumference was
measured to be 52.4 cm and a cruising altitude circumference
was measured to be 54.9 cm. Assuming a spherical balloon and
that the ground pressure was 1.00 atm, the pressure at cruising
altitude would be 0.869 atm.
-
- Using the relationship between altitude and pressure described
on p.26 of van Loon and Duffy (van Loon, G. W.; Duffy, S. J.
Environmental Chemistry: A Global Perspective, Oxford
University Press, 2000), a pressure of 0.869 atm relative to
a sea-level pressure of 1.00 atm corresponds to an altitude of
4000 feet. Most planes I have been on cruise at a around 35,000
feet, which corresponds to a pressure of 0.294 atm relative to
a sea-level pressure of 1.00 atm. Clearly the cabin of the plane
is pressurized!


ABOVE: Sealed roll package on an airplane (LEFT) at ground level
and (RIGHT) in the air.
- Balloon Gas Effusion (posted 3-18-12)
-


-
- ABOVE: (LEFT) Balloons initially filled to the same volume with nitrogen and helium gases. (RIGHT) Helium gas (4 g/mol) leaves its balloon faster than nitrogen gas leaves its balloon (28 g/mol).
- Turning Off Glowing Toys (posted 12-4-11)
- Most glow-in-the-dark toys glow green due to zinc sulfide doped with elements such as copper. In about 1998, I was playing with a red laser pointer and some powdered doped zinc sulfide phosphor and found that the red laser cannot stimulate the phosphor to glow. The red light did not have enough energy to promote the phosphor electrons to the excited state. However, when the phosphor was already excited and glowing green and then was irradiated with the red laser the phosphor briefly glowed more brightly, and then darkened. I could use the red laser to draw lines of darkeness on the glowing doped zinc sulfide. I have found over the years that this stimulated emission and darkening phenomenon works using red light on other glow-in-the-dark objects, including glowing drawing screens sold by toy companies or the glowing screens at museums that record people's shadows from a light flash. I think this ability to use light to draw dark areas or lines on glowing objects could be used to enhance the utility/enjoyment of these materials. I would like to know more details about the mechanisms behind this phenomenon and welcome any insights that you might have.



ABOVE: A glow-in-the-dark butterfly shape gives a butterfly-shaped glow (LEFT), but when irradiated with a red laser pointer on the middle the glow is darkened (MIDDLE) or irradiated with a red laser pointer on the left wing the glow is darkened (RIGHT). Sorry the pictures are so faint - the glow was difficult to capture with my camera and you might have to adjust the angle of your viewing screen.
- Chelate Effect with a Bag Closure (posted 3-18-12)
- Polydentate ("chelating") ligands have more than one point of connection to a metal center and tend to bind more strongly to those metals than monodentate ligands (which only have more than one point of connection). This can be illustrated with a plastic bag closure (see: http://www.kwiklok.com/kwik-lok-bag-closures.php). The two hook-like edges of the closure hold the bag closed more effectively than half a closure, which has only one hook.
-


ABOVE: The two hook-like edges of a bag closure (LEFT) hold the bag closed more effectively than half a closure, which has only one hook (RIGHT).
Return to Dr. Campbell's Favorite
Demonstrations
Last updated 3/18/12
Site created at the laboratory of Dean Campbell














