Demonstrations Page 7 - Redox
Scroll down to view photographs and short descriptions of
some of the demonstrations in the list below.
WARNING – Use at your own risk! We cannot guarantee the accuracy or the safety of these activities. Some of these activities are far more dangerous than others. The contributors and Bradley University do not assume any responsibility for these activities or their results. If you have questions, corrections, or comments please do not hesitate to contact Dean Campbell (campbell@bumail.bradley.edu) at Bradley University.
- Ferrofluid
Demonstrations
- Refrigerator
Magnet Demonstrations
- Polydimethysiloxane
Demonstrations
- LEGO® Brick Chemistry and Nanotechnology Demonstrations
Vitamin C Cleans Up Iodine Stains on
Hand
- I learned this demo from a visitor to campus a couple years
ago. This demo takes only a couple minutes. To put this together,
you need to go to the local drugstore/grocery store and pick
up the following:
- -iodine solution (tincture of iodine, used as a disinfectant)
- -spray starch
- -vitamin C tablets
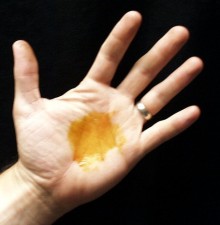
- Step 1 - Smear some of the iodine solution on the palm of
your hand. DO NOT let the iodine solution dry out (otherwise
it will be hard to remove).
- Step 2 - While the iodine solution is still wet, spray some
starch onto the palm of your hand. The iodine spot turns blue-black
as the starch molecules wrap around the iodine molecules. Again,
DO NOT let this mess on your hand dry out.
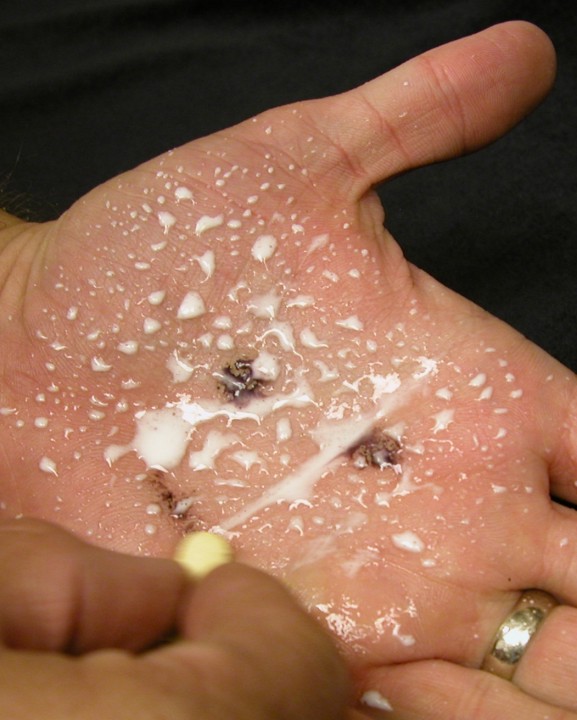
- Step 3 - While the iodine/starch solution is still wet, take
a vitamin C tablet and rub it across the stain (I make a smiley
face first). The dark stain disappears though sometimes there
might still be some yellow tint left. The vitamin C tablet acts
as an antioxidant, reducing the iodine to iodide ions and breaking
apart the starch/iodine complex. The tablet itself stays fairly
white, this it not simply rubbing the stain off, it is a chemical
reaction.
- CAUTIONS:
- Wash your hands with soap and water as soon as you get the
chance after the demo (the starch on your hand will still be
sticky/slimy).
- Iodine stains all sorts of things, so do not spill it.
- DO NOT eat the vitamin C tablet that you used in the demo,
though you should be able to use the same tablet multiple times.
- You should try this demo by yourself first in case I missed
any subtle details.
- VARIATION: A counterfeit money marking pen, which apparently
contains iodine, can be used instead of tincture of iodine. The
iodine in the pen does not react with the cloth fibers in real
money, but it does react with the cellulose fibers in counterfeit
money. The pen can be used to draw light tinted patterns on skin,
but the markings dry out fairly quickly so the starch must be
applied quickly to turn them dark. These starch/iodine marks
can also be erased with a vitamin C tablet.

- ABOVE: (LEFT) Step 1 (MIDDLE) Step 3 and (RIGHT) Variation:
Erasing counterfeit money pen marks.
-
- Coin Batteries
- These batteries are simply voltaic piles made by stacking
alternating types of coins with filter paper soaked in saturated
salt water solution. The metal compositions of pennies are sufficiently
different from nickels so that these coins may be used to make
weak batteries. More coins can produce more voltage, but there
is a tremendous variability in the actual voltage measured. This
is likely due to effects such as internal resistance and degree
of corrosion on the coins.
- See: Scharlin, P.; Battino, R.; Boschman, E. J. Chem.
Educ. 1991, 68, 665.
-



- ABOVE: (LEFT) A simple coin battery - a nickel on salt-water
soaked filter paper on a penny. (MIDDLE) Measuring the potential
of a single pair of coins (in millivolts). (RIGHT) Measuring
the potential of multiple pairs of coins (in millivolts).
- Iron Filings in a Sealed Bottle
- A couple of years ago I placed coarse iron powder in a sealed
bottle to use in magnetism demonstrations. Recently I noticed
that the bottle had partially collapsed and hypothesized that
it the oxygen in the bottle's air had oxidized the iron to produce
iron oxide. I used the volume displacement method (a large graduated
cylinder partially filled with water) to estimate the gas volume
in the collapsed bottle (405 mL) and in the bottle after allowing
air to refill the bottle (490 mL). This represents a percent
gas volume change of 17%, which is in the ballpark of the typical
21% oxygen concentration for air.
- See: Campbell, D. J.; Bannon, S. J.; Gunter, M. M. J. Chem. Educ., 2011, 88, 784-785.
-
-


- ABOVE: (LEFT) Partially collapsed bottle containing iron
powder. (RIGHT) Reinflated bottle containing iron powder.
- Chemiluminesent Reaction
- This reaction involves a water/acetonitrile solvent, tris(bipyridine)ruthenium(II) ions, ammonium persulfate, and magnesium metal. During the course of the electron transfers in this system, the excited ruthenium complex emits an orange glow near the magnesium metal, producing the appearance of hot coals in the bottom of the reaction vial.
- See: White, H. S.; Bard, A. J. J. Am. Chem. Soc., 1982, 104, 6891.
-
-
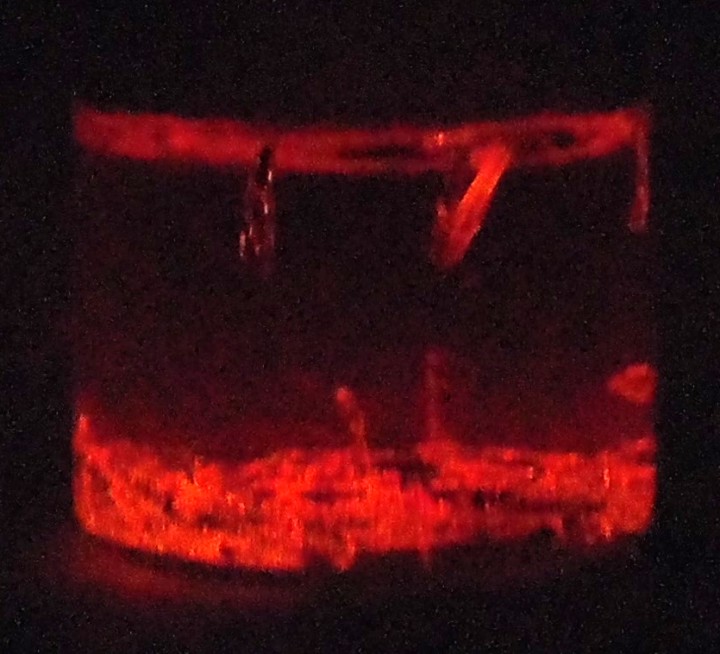
- ABOVE: Pretty orange chemiluminescence.
-
-
- Return to Dr. Campbell's Favorite
Demonstrations
Last updated 2/14/12
Site created at the laboratory of Dean Campbell















